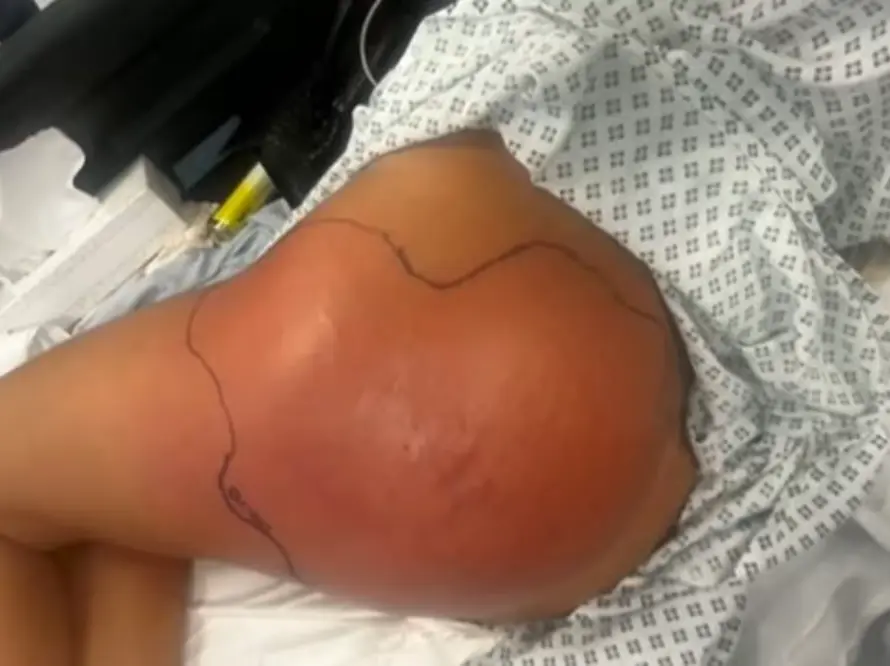
Suppliers of unlicensed botulinum toxins face a prison sentence
Save Face has long highlighted public safeguarding from fake ‘Botox’-like injectables and unlicensed botulinum toxin brands, which cannot legally be used in the United Kingdom. This work is part of numerous high-profile campaigns targeting regulators and legislators.
We are pleased to support the crackdown, announced by the Medicines and Healthcare products Regulatory Agency (MHRA) at the end of the summer, aimed at tackling the illicit trade in unlicensed botulinum toxins. The MHRA notes that ‘criminals’ caught in this trade - selling or supplying - will face up to two years in prison and unlimited fines under the Human Medicines Regulations 2012.[i]
At Save Face, we have seen a dramatic increase in complaints related to illegal products under the ‘Botox’ umbrella. These include counterfeits of licensed brands or substances claimed to be botulinum toxin yet found to be something entirely different. However, most recent complaints relate to neurotoxin brands not approved for use in the UK. These brands are often South Korean and widely promoted via social media.
Botulinum toxin brands currently licensed in the UK for aesthetic procedures include Alluzience®, Azzalure®, Bocouture®, Botox®, Letybo®, Nuceiva® and Relfydess®.
The number of ‘Botox’ related complaints received by Save Face has increased by over 350% during the last five years, from 2019 to 2024.[ii] Across all reporting years, most complainants, over 80%, were unaware that botulinum toxin is a prescription-only medicine, were treated by a non-healthcare practitioner, did not have a face-to-face consultation with a licensed prescriber, and did not know which product was ultimately used. Similarly, many practitioners were unwilling to supply prescription records, lot numbers, or details of the products they used when challenged following a complaint by a member of the public. At least a third of the complaints were believed to be related to unlicensed or counterfeit botulinum toxin products, whose potency can vary.
The recent response from the MHRA follows a public health episode. After a spike in hospital admissions, predominantly across the northern and eastern regions of England between June and August 2025, the MHRA’s Criminal Enforcement Unit (CEU) launched several criminal investigations. 41 confirmed cases of iatrogenic botulism were reported by the UK Health Security Agency (UKHSA).[iii],[iv] Those affected, both men and women, suffered reactions including slurred speech, difficulty swallowing, and breathing difficulty that required respiratory support, with 8 patients needing intensive care treatment. Due to the unprecedented influx of cases across the north, hospitals rushed to secure additional stocks of anti-toxin from national health service providers around the country.[v] Where the name of the injectable product was known to the individual, most were reported to be unlicensed products manufactured in South Korea, but many did not know the name of the injectable used.[vi] The BBC, having spoken to several victims, quoted a number as having been treated with Toxpia.[vii]
The CEU has reported seeing evidence that some sellers are working within organised, criminal networks, and practitioners, who are often untrained, are obtaining unlicensed botulinum toxin products illegally, and offering injections in unsafe, unregulated settings, including domestic bedrooms and kitchens via mobile beauty services. Members of the public are often drawn in by social media advertising offering cheap, unrealistic deals.
The crackdown is part of the MHRA’s wider work to disrupt illegal botulinum toxin supply. Since May 2023, the CEU, working closely with its partners in Border Force, has seized more than 4,700 vials of unlicensed botulinum toxin (unapproved for use in the UK), both at the border and inland, including brands originating from South Korea such as Botulax, reNTox, Innotox, and Toxpia.
As champions of public messaging to raise awareness surrounding seeking aesthetic treatments safely, including botulinum toxins, the team at Save Face is heartened to see that the official press release from the MHRA, distributed to widespread media outlets, has signposted the public to seek out medically qualified practitioners. Their statement reads,
“Licensed botulinum toxin products undergo rigorous testing and quality controls to ensure they contain the correct active ingredient at safe concentrations. Legitimate treatments should only be carried out by qualified healthcare professionals in proper clinical settings with appropriate emergency equipment available.
Before any treatment, verify that your practitioner is medically qualified and registered with their professional body. Check that products being used are licensed in the UK by asking to see packaging and checking batch numbers. Be suspicious of unusually cheap prices, treatments offered in domestic settings, or practitioners who cannot provide proper credentials.”i
Although rare, cases of iatrogenic botulism following cosmetic injection of botulinum toxin are becoming more widely reported around the globe, with some published studies highlighting controversial and illegal practices. A plastic surgery team in China published a retrospective study in August 2025. It identified 161 cases of cosmetic iatrogenic botulism reported in a large, single centre over ten years, between 2014 and 2024. The cases featured an even split between hospitalisation and outpatient intervention; most cases, however, occurred in 2024, showing a troubling increase.
The authors found that, ‘most patients received botulinum toxin injections of unknown origin from unlicensed practitioners in nonmedical settings.’ They regarded it as ‘the largest reported outbreak of illicit cosmetic iatrogenic botulism worldwide’ and pointed to the risks associated with counterfeit botulinum toxin and noncompliant practices. They underscored the critical need for stringent surveillance of botulinum toxin distribution.[viii]
At Save Face, we support the MHRA in drawing attention to the criminals involved in the supply and administration of unlicensed botulinum toxins who are exploiting the popularity of cosmetic treatments, breaking the law, and peddling dangerous, unapproved products. They are endangering lives, as proven by the recent cases of hospitalisation, and putting profit before any thought for public safety. We will continue to report cases that come to our attention to the authorities, work with regulators to stamp out supply, and assist enforcement processes.
References
[i] MHRA crackdown on illegal ‘Botox’ after victims left seriously ill (published 30th August 2025) - https://www.gov.uk/government/news/mhra-crackdown-on-illegal-botox-after-victims-left-seriously-ill
[ii] Internal Data at Save Face – 5 Year Botox Related Complaints
[iii] UKHSA issues warning over botulism (published 18th July 2025) - https://www.gov.uk/government/news/ukhsa-issues-warning-over-botulism
[iv] Iatrogenic botulism outbreak associated with aesthetic procedures using injectable botulinum toxin products. (HPR volume 19 issue 8: news (7, 14 and 28 August 2025) - https://www.gov.uk/government/publications/health-protection-report-volume-19-2025/hpr-volume-19-issue-8-news-7-august-2025
[v] BBC - 'I was poisoned by fake Botox' (published 20th June 2025) - https://www.bbc.co.uk/news/articles/cn0q2z725llo
[vi] UKHSA - Iatrogenic botulism associated with cosmetic botulinum toxin injections (Serial number: BN2025/029, Date: 18/07/2025 ) https://cptv.org.uk/wp-content/uploads/sites/109/2025/07/20250718_BN2025_29_Iatrogenic-botulism-associated-with-cosmetic-botulinum-toxin-injections_v01.00.pdf
[vii] BBC - Women poisoned by fake Botox get apology from beautician (published 3 July 2025) https://www.bbc.co.uk/news/articles/c89eey7jjeno
[viii] Qiu H, Shen J, Tang Y, Ji Q, Lin X, Wu D. A Retrospective Case Series Study of Illegal Cosmetic Iatrogenic Botulism: Outbreak Analysis and Response Lessons. Aesthet Surg J. 2025 Aug 18;45(9):936-946. doi: 10.1093/asj/sjaf088. PMID: 40391523. https://pubmed.ncbi.nlm.nih.gov/40391523/